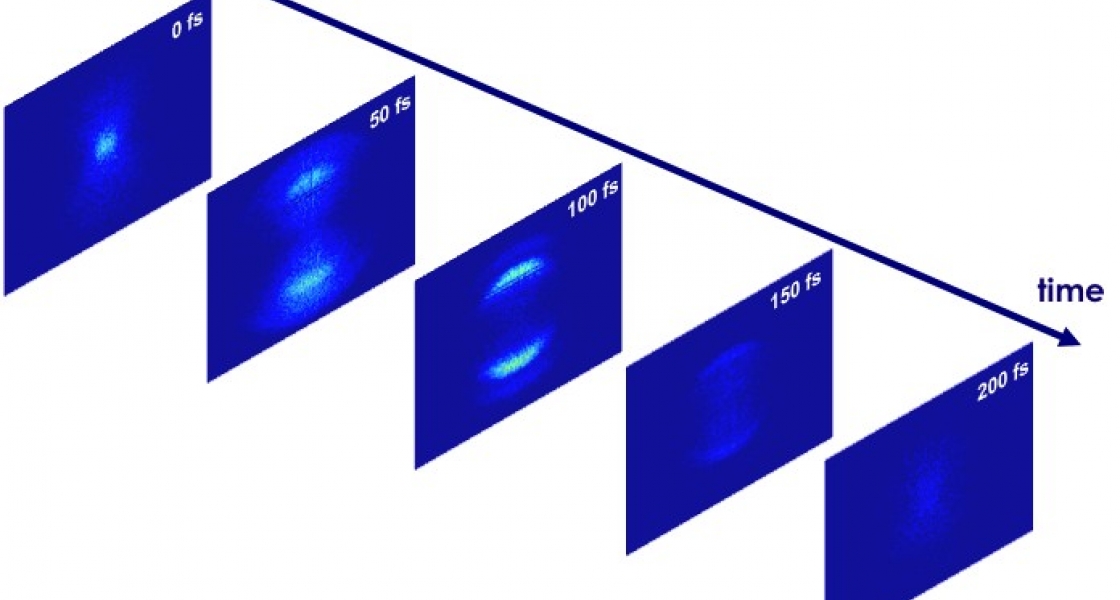
The dance of electrons as a bromine molecule (Br2) separates into two atoms is intricate and complex. The process of breaking up takes far longer than expected (~150 vs 85 fs) because the cloud of electrons that bind atoms together in a molecule behaves as if it were still surrounding a molecule until the last possible moment — when the atomic fragments are about twice the normal distance apart (~.55 nm). At this point, there’s simply not enough energy left in the system to hold the molecule together. When the two atoms finally appear as separate entities, it was if someone had snapped a rubber band.
Bromine molecule’s long goodbye was discovered in an experiment by former research associate Wen Li of the Kapteyn/Murnane group and explained theoretically by senior research associate Agnieszka Jaron-Becker of the Becker group. Graduate student Craig Hogle, former research associates Vandana Sharma and Xibin Zhou, and Fellows Andreas Becker, Henry Kapteyn, and Margaret Murnane contributed to this seminal work.
Li and his colleagues initiated the dissociation of a Br2 molecule with a violet (400 nm) ultrafast laser pulse. If a Br2 molecule absorbs only a single photon at this wavelength, the molecule gets excited into a dissociative state where it begins to break apart into its constituent atoms. The researchers then used an infrared laser pulse to ionize the dissociating molecule and probe the progression of the dissociation at varying times after the molecule began to fall apart. The series of probe pulses allowed Li and his colleagues to follow the motions of all ten valence electrons as the molecular bond was breaking. This is the first experiment to capture the coordinated dance of multiple electrons at once.
What surprised the team was that the change from a molecule to two atoms could be observed over such a surprisinglylong time and distance. (While times of 140 millionths of a billionth of a second and distances of .55 billionths of a meter may seem very small, they are much longer than would normally be expected in the exceedingly fast and small world where individual atoms interact.) To explain these unexpected results, the laboratory scientists turned to their theorist colleagues.
With the help of Jaron-Becker, the laboratory scientists realized they were observing all 10 valence electrons evolving in time as the molecular bond was breaking. Jaron-Becker’s analysis showed that the researchers were not likely to see two separate atoms until 140–160 fs after the initial dissociative laser pulse — a prediction that dovetailed nicely with the experimental observations.
“What was interesting about this system was that there was a single excited initial state, but 30 possible final states, with many different ways to get there,” said Jaron-Becker. She recalled that the experiment was already underway when she arrived at JILA. At that time, the Kapteyn/Murnane group thought that in Br2, they had selected something nice to study, but not too difficult.
“But when we first looked at Br2’s potential energy surfaces, we thought ‘Oh, God, that’s a mess,’ ” said Jaron-Becker’s husband and colleague Andreas Becker. Jaron-Becker said it took her half a year to get the potential energy surfaces and corresponding wave functions with sufficient accuracy to allow her to calculate the ionization rates of the Br2molecule. “But when we got the figures, we discovered they looked like the experimental data,” Jaron-Becker said.
The Beckers believe the Kapteyn/Murnane group picked about the most difficult diatomic molecule for a theoretical analysis that they could have chosen. “But they did a beautiful experiment and saw what Agnieszka’s calculations predicted they would observe,” Becker said. He added that this work opens a new door in the theory of molecular dissociation. It is a proof of principle for observing and understanding electron rearrangements during molecular dissociations over long times and distances. - Julie Phillips
Watch a movie of a bromine molecule falling apart after being hit with an ultrafast laser!


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.